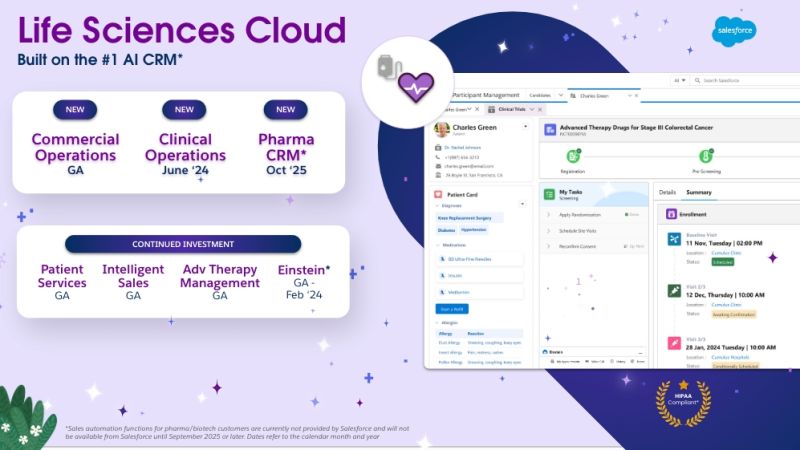
How Cereblis Can Elevate the Potential of Salesforce’s New Life Sciences Cloud Launch for Clients
Salesforce's Life Sciences Cloud is set to be a game-changer for the industry. But like any tool, its success in a company hinges on its implementation and adoption. With Cereblis's expertise in both Salesforce and the life sciences sector, clients are assured of a partner who understands their challenges and can guide them to success in this new digital era.