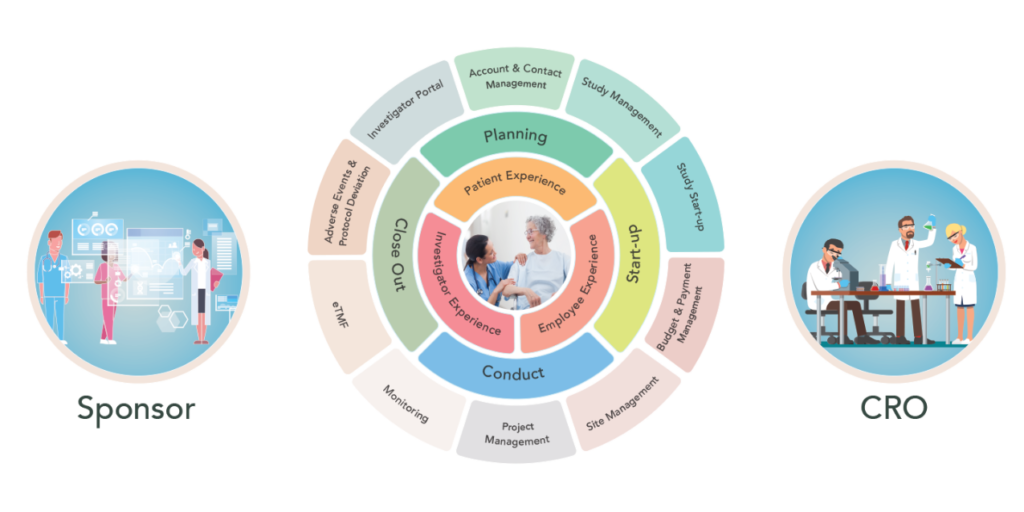
In the rapidly evolving field of diagnostics, the ability to manage complex clinical trials efficiently and in compliance with regulatory requirements is paramount. Diagnostics companies face unique challenges in clinical trial management, from stringent regulatory oversight to the need for precise data collection and analysis. Cloudbyz Clinical Trial Management System (CTMS) is specifically designed to address these challenges, making it the best choice for diagnostics companies. Here’s why Cloudbyz CTMS stands out as the premier solution for managing diagnostics clinical trials.
1. Specialized Support for Diagnostic Trials
Diagnostic trials differ from therapeutic trials in several key ways, including the need for rigorous validation processes, multi-site coordination, and strict regulatory compliance. Cloudbyz CTMS is tailored to meet these specific needs, offering robust support for the entire lifecycle of diagnostic trials, from feasibility and site selection to post-market surveillance.
Cloudbyz CTMS includes features specifically designed to manage the intricacies of diagnostic trials, such as real-time data integration, automated workflows, and comprehensive documentation management. These tools help streamline the trial process, reduce administrative burden, and ensure that all regulatory requirements are met.
2. Compliance with Global Regulatory Standards
Diagnostics companies must navigate a complex landscape of global regulations, including the FDA’s 21 CFR Part 11, the EU’s In Vitro Diagnostic Regulation (IVDR), and other international standards. Cloudbyz CTMS is built with these regulatory requirements in mind, providing tools to ensure compliance at every stage of the trial.
The platform offers robust audit trails, electronic signatures, and automated reporting to help diagnostics companies maintain compliance with global standards. By ensuring that all regulatory documentation is accurate, up-to-date, and easily accessible, Cloudbyz CTMS reduces the risk of compliance issues and accelerates the approval process.
3. Integrated Data Management and Analytics
One of the most critical aspects of diagnostic trials is the management and analysis of large volumes of data. Cloudbyz CTMS excels in this area by providing integrated data management and advanced analytics tools that enable real-time insights and data-driven decision-making.
The platform’s data integration capabilities allow for seamless connectivity with various diagnostic instruments, electronic data capture (EDC) systems, and laboratory information management systems (LIMS). This ensures that all trial data is captured, stored, and analyzed within a single, unified platform, providing a comprehensive view of trial progress and outcomes.
Cloudbyz CTMS also offers customizable dashboards and reporting tools, allowing diagnostics companies to track key performance indicators (KPIs), monitor trial metrics, and generate real-time reports. These features are essential for ensuring that trials are conducted efficiently and that data is analyzed accurately and promptly.
4. Streamlined Study Planning and Site Management
Effective study planning and site management are crucial for the success of diagnostics trials, which often involve multiple sites and complex logistics. Cloudbyz CTMS offers comprehensive tools for study planning, site selection, and site management, helping diagnostics companies optimize trial operations and ensure that all sites are performing at their best.
The platform’s site management features include site performance tracking, contract management, and budget management, allowing for efficient coordination of multi-site trials. Cloudbyz CTMS also provides automated workflows and task management tools, which streamline communication and ensure that all trial activities are completed on time and within budget.
5. Enhanced Collaboration and Communication
Collaboration between stakeholders, including sponsors, CROs, sites, and regulatory bodies, is essential for the success of diagnostics trials. Cloudbyz CTMS provides a centralized platform for communication, document sharing, and collaboration, ensuring that all stakeholders have access to the information they need and can work together effectively.
The platform’s role-based access controls ensure that each stakeholder has access to the appropriate level of information, maintaining data security while promoting transparency and accountability. By facilitating effective collaboration, Cloudbyz CTMS helps diagnostics companies manage complex trials more efficiently and achieve better outcomes.
6. Flexibility and Scalability for Diagnostics Trials
Diagnostics trials can vary greatly in scope and complexity, from small-scale validation studies to large, multi-center clinical trials. Cloudbyz CTMS is designed to be flexible and scalable, allowing diagnostics companies to tailor the platform to meet the specific needs of each trial.
Whether managing a small, single-site study or a large, global trial, Cloudbyz CTMS can be customized to fit the unique requirements of diagnostics trials. The platform’s cloud-based architecture ensures that it can scale to accommodate trials of any size, providing a long-term solution that grows with the company’s needs.
7. Real-Time Monitoring and Risk Management
In diagnostics trials, the ability to monitor trial progress in real-time and manage potential risks is critical. Cloudbyz CTMS offers advanced monitoring and risk management tools that provide real-time visibility into trial activities, enabling proactive risk management and timely decision-making.
The platform’s real-time monitoring capabilities allow clinical teams to track patient enrollment, site performance, and data collection in real-time, identifying potential issues early and taking corrective action as needed. This proactive approach to risk management helps ensure that diagnostics trials are conducted smoothly and that any potential risks are mitigated before they impact trial outcomes.
8. Cost-Effective Trial Management
Managing the costs of clinical trials is a significant concern for diagnostics companies, where budget constraints can impact trial timelines and outcomes. Cloudbyz CTMS offers cost-effective trial management solutions that help diagnostics companies optimize their budgets and resources.
The platform’s budget management tools allow for accurate forecasting, cost tracking, and resource allocation, ensuring that trials are completed on time and within budget. Cloudbyz CTMS also provides tools for managing contracts, payments, and invoicing, helping diagnostics companies control costs and maximize the return on their investment in clinical research.
9. Comprehensive Post-Market Surveillance
Post-market surveillance is a critical component of diagnostics trials, particularly for in vitro diagnostics (IVD) that are already on the market. Cloudbyz CTMS provides robust tools for managing post-market surveillance activities, including adverse event reporting, safety monitoring, and ongoing data collection.
The platform’s post-market surveillance features help diagnostics companies ensure that their products continue to meet safety and efficacy standards after they have been brought to market. By centralizing post-market data and automating reporting processes, Cloudbyz CTMS helps diagnostics companies maintain compliance with regulatory requirements and protect patient safety.
10. Dedicated Customer Support and Expertise
Diagnostics companies require specialized support and expertise to navigate the complexities of clinical trial management. Cloudbyz CTMS offers dedicated customer support services, ensuring that diagnostics companies receive the assistance they need at every stage of the trial process.
From implementation and training to ongoing support and optimization, Cloudbyz’s team of experts is available to help diagnostics companies maximize the value of their CTMS investment. This commitment to customer success, combined with the platform’s powerful features and capabilities, makes Cloudbyz CTMS the best choice for managing diagnostics clinical trials.
11. Comprehensive Study Startup and Site Activation
One of the most time-consuming aspects of clinical trials is study startup and site activation. This is particularly true in diagnostics trials, where multiple sites may need to be onboarded quickly and efficiently. Cloudbyz CTMS streamlines the study startup process with automated workflows, document management, and site selection tools that reduce the time and effort required to get sites up and running.
The platform’s site activation features include contract and budget management, which ensure that all necessary agreements are in place before the trial begins. Cloudbyz CTMS also provides tools for tracking site readiness and performance, enabling trial managers to monitor progress and address any issues that arise during the activation process.
By accelerating study startup and site activation, Cloudbyz CTMS helps diagnostics companies reduce time to market and ensure that trials begin on schedule.
12. Support for Adaptive and Innovative Trial Designs
The diagnostics industry is increasingly embracing adaptive trial designs, which allow for modifications to the trial protocol based on interim results. These innovative trial designs can lead to faster and more cost-effective development of diagnostic tests, but they also require flexible and robust trial management systems.
Cloudbyz CTMS is ideally suited to support adaptive and innovative trial designs, offering features that enable real-time data analysis, protocol amendments, and adaptive decision-making. The platform’s flexibility ensures that diagnostics companies can implement adaptive trials with confidence, knowing that their CTMS can handle the complexities of these designs.
13. Integration with Emerging Technologies
As the diagnostics industry continues to innovate, new technologies such as artificial intelligence (AI), machine learning (ML), and digital biomarkers are playing an increasingly important role in clinical trials. Cloudbyz CTMS is designed to integrate with these emerging technologies, providing a future-proof solution that can adapt to the changing landscape of diagnostics research.
The platform’s ability to integrate with AI and ML tools enables diagnostics companies to leverage advanced analytics and predictive modeling to enhance trial outcomes. Additionally, Cloudbyz CTMS supports the use of digital biomarkers and remote monitoring technologies, which are becoming increasingly important in diagnostics trials.
By integrating with these emerging technologies, Cloudbyz CTMS ensures that diagnostics companies remain at the forefront of innovation and can take advantage of the latest advancements in clinical research.
14. Enhanced Patient Engagement and Retention
Patient engagement and retention are critical challenges in diagnostics trials, particularly in studies that require frequent testing or long-term follow-up. Cloudbyz CTMS offers a range of features designed to enhance patient engagement, including electronic patient-reported outcomes (ePRO), patient portals, and automated communication tools.
These features help keep patients informed and engaged throughout the trial, improving retention rates and ensuring that data is collected consistently and accurately. Cloudbyz CTMS also supports the use of decentralized trial models, which can make it easier for patients to participate in trials from the comfort of their own homes.
By enhancing patient engagement and retention, Cloudbyz CTMS helps diagnostics companies collect high-quality data and achieve better trial outcomes.
15. Comprehensive Data Security and Privacy Controls
In diagnostics trials, the protection of sensitive patient data is of utmost importance. Cloudbyz CTMS is built with comprehensive data security and privacy controls that ensure all trial data is protected from unauthorized access and breaches.
The platform complies with global data protection regulations, including GDPR and HIPAA, and offers encryption, role-based access controls, and audit trails to safeguard data. Cloudbyz CTMS also provides tools for managing data access and permissions, ensuring that only authorized personnel have access to sensitive information.
By providing robust data security and privacy controls, Cloudbyz CTMS helps diagnostics companies maintain the highest standards of data integrity and confidentiality.
Conclusion
In the competitive and highly regulated diagnostics industry, having the right Clinical Trial Management System is critical to ensuring the success of clinical trials. Cloudbyz CTMS stands out as the best choice for diagnostics companies, offering a comprehensive, flexible, and integrated platform that meets the unique needs of diagnostics trials. From regulatory compliance and integrated data management to real-time monitoring and cost-effective trial management, Cloudbyz CTMS provides the tools and support needed to conduct efficient, compliant, and successful diagnostics trials.
For diagnostics companies looking to streamline their clinical trial management, reduce time to market, and achieve better outcomes, Cloudbyz CTMS is the solution that delivers. Its specialized features, flexibility, and ability to integrate with emerging technologies make it the best choice for managing the complexities of diagnostics clinical trials today and in the future.

