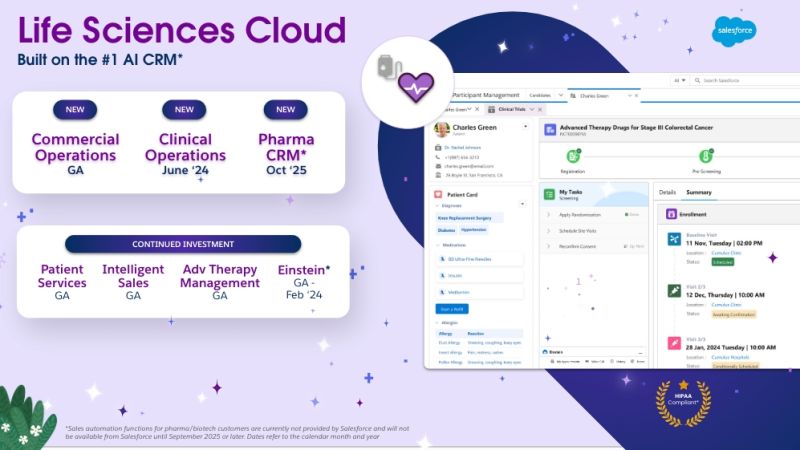
How AI Can Enhance Clinical Data Management
Cloudbyz EDC is a powerful tool that enhances the quality, compliance, and efficiency of clinical trials. Its user-friendly interfaces, robust collaboration features, and comprehensive data security measures make it an ideal choice for organizations seeking to streamline their clinical research processes. By leveraging Cloudbyz EDC, researchers can ensure that their data is accurate, compliant, and secure, ultimately contributing to the success of their clinical trials.