

FEATURED BLOG

Why Cloudbyz CTMS is the Best Choice for Biotechnology Companies
For biotechnology companies looking to streamline their clinical trial management, reduce time to market, and achieve better outcomes, Cloudbyz CTMS is the solution that delivers. Its specialized features, scalability, and commitment to innovation make it the best choice for managing the complexities of biotechnology clinical trials today and in the future.

Why Cloudbyz CTMS is the Best Choice for Biotechnology Companies
For biotechnology companies looking to streamline their clinical trial management, reduce time to market, and achieve better outcomes, Cloudbyz CTMS is the solution that delivers. Its specialized features, scalability, and commitment to innovation make it the best choice for managing the complexities of biotechnology clinical trials today and in the future.

FEATURED BLOG
September 25, 2020 by Ani Mayasandra
We analyzed 164 pharmacovigilance startups impacting the industry. HEPAprint, MEDIKURA, Navro, Embleema & Cloudbyz develop 5 top solutions to watch out for. Learn more in our Global Startup Heat Map!
Blog > Digital Services

How AI Can Enhance Clinical Data Management
Cloudbyz EDC is a powerful tool that enhances the quality, compliance, and efficiency of clinical trials. Its user-friendly interfaces, robust collaboration features, and comprehensive data security measures make it an ideal choice for organizations seeking to streamline their clinical research processes. By leveraging Cloudbyz EDC, researchers can ensure that their data is accurate, compliant, and secure, ultimately contributing to the success of their clinical trials.

Ensuring Compliance with Health Canada’s Foreign Actions Profile: A Digital Solution
Navigating the complexities of Health Canada’s Foreign Actions Profile requirements necessitates a proactive and integrated approach. By leveraging digital solutions, pharmaceutical companies can streamline their compliance processes, reduce the risk of non-compliance, and ensure the safety and efficacy of their products in the Canadian market. Embracing these technologies not only enhances regulatory adherence but also promotes operational efficiency and risk management, positioning companies for success in an increasingly regulated global environment.

Transforming Patient Support and Access Programs in Pharmaceutical Companies with Salesforce Health Cloud
In an era where patients are increasingly taking an active role in managing their healthcare, pharmaceutical companies must adapt by offering patient-centric support and access programs. Salesforce Health Cloud provides the tools and capabilities necessary to transform patient support and access, enabling pharmaceutical companies to deliver personalized care, engage with patients effectively, and streamline access initiatives. Embracing this CRM platform can not only improve patient outcomes but also enhance the reputation and competitiveness of pharmaceutical companies in the evolving healthcare landscape.
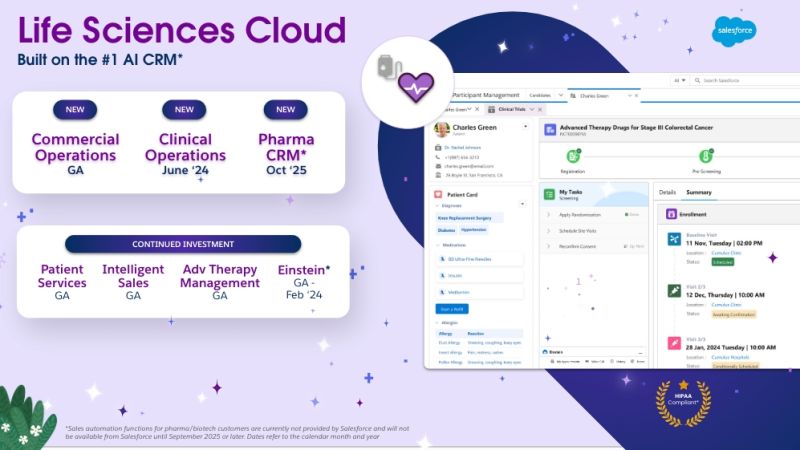
How Cereblis Can Elevate the Potential of Salesforce’s New Life Sciences Cloud Launch for Clients
Salesforce’s Life Sciences Cloud is set to be a game-changer for the industry. But like any tool, its success in a company hinges on its implementation and adoption. With Cereblis’s expertise in both Salesforce and the life sciences sector, clients are assured of a partner who understands their challenges and can guide them to success in this new digital era.

25 Reasons Why Pharmaceutical Companies Need a Unified Clinical Trial Management Platform Built on Salesforce
A unified clinical trial management platform built on Salesforce offers a versatile and powerful solution for pharmaceutical companies. With its scalability, regulatory compliance, and robust features, Salesforce empowers organizations to streamline their clinical trial operations, reduce costs, and accelerate drug development. By embracing this platform, pharmaceutical companies can not only enhance their efficiency but also contribute to the timely delivery of life-saving medications to patients worldwide.

Investigator Initiated Trials (IIT), Investigator Initiated Research (IIR), and Grants Management: Digitization with Salesforce Experience Cloud
Investigator Initiated Trials (IIT), Investigator Initiated Research (IIR), and Grants Management are vital components of advancing medical research. Salesforce Experience Cloud offers a comprehensive solution for digitizing and streamlining these processes, ensuring efficient management, compliance, and collaboration among stakeholders. By harnessing the power of Salesforce Experience Cloud, research institutions can accelerate the pace of discovery and make a meaningful impact on patient care and scientific knowledge.

Unlocking the Power of Patient Services Program Capabilities
Patient services programs are a vital component of modern healthcare, designed to enhance patient experiences and outcomes. Their capabilities extend from personalized support and education to financial assistance, care coordination, and leveraging technology for remote monitoring. These programs play a pivotal role in improving the overall quality of care, fostering patient engagement, and ensuring that individuals receive the support they need to lead healthier lives. As the healthcare landscape continues to evolve, patient services programs will remain a cornerstone of patient-centered care.

Transforming Patient Support and Access Programs in Pharmaceutical Companies with Salesforce Health Cloud
In an era where patients are increasingly taking an active role in managing their healthcare, pharmaceutical companies must adapt by offering patient-centric support and access programs. Salesforce Health Cloud provides the tools and capabilities necessary to transform patient support and access, enabling pharmaceutical companies to deliver personalized care, engage with patients effectively, and streamline access initiatives. Embracing this CRM platform can not only improve patient outcomes but also enhance the reputation and competitiveness of pharmaceutical companies in the evolving healthcare landscape.

Challenges of managing PHI & PII Data in Life Sciences and How AI/ML can help
The challenge of managing and securing sensitive data in life sciences organizations is significant and growing. However, AI/ML-based PHI and PII solutions offer a promising solution to these challenges, helping organizations protect sensitive data, comply with data protection regulations, and improve efficiency and accuracy.

Best Practices in Managing Medical Information in Pharmaceutical Companies
Effective management of medical information is a cornerstone of pharmaceutical companies’ responsibilities to provide accurate, timely, and compliant information to healthcare professionals and patients. Implementing best practices in this area not only ensures regulatory compliance but also fosters trust and transparency within the healthcare ecosystem. By centralizing processes, adhering to SOPs, and continuously monitoring and improving operations, pharmaceutical companies can enhance their medical information management capabilities and ultimately contribute to better patient care and safety.
Market Access Development Programs: Accelerating Pharmaceutical Drug Commercialization
Market access development programs are essential for the successful commercialization of pharmaceutical drugs. These programs encompass a range of strategies and activities aimed at overcoming barriers to market entry, ensuring patient access, and optimizing pricing and reimbursement. By effectively navigating the complex landscape of drug commercialization, market access programs play a pivotal role in bringing innovative medications to patients and improving global healthcare outco
Investigator-Initiated Trials Project Development: Critical Questions
Investigator-initiated trials are a valuable avenue for advancing medical knowledge and improving patient care. By addressing these critical questions during the project development phase, you can increase the likelihood of success and contribute to the growth of clinical research. Remember that collaboration, meticulous planning, and a commitment to ethical research are essential elements of a successful IIT project.
Cereblis enables companies to take advantage of innovation in tech by onboarding new ways to work and execute projects by advising, implementing, and optimizing digital solutions.
Cereblis LLC, 1770, Park Street,
Suite 108, Naperville IL 60563.
Phone: +1 (312)-763-8040
Email: info@cereblis.com