Clinical Trials, slowly but surely are becoming the new talking point of our generation. Even the teenagers of this contemporary age were discussing and searching about the clinical trials over the internet. Many of the world’s powerful bureaucracies are closely monitoring the curiosity spinning around clinical trials.
What’s the basis for such significance?
The planet slowed to a standstill because of the COVID-19 and the global economy is at its highest peril. The only ray of hope is the ongoing clinical trials for the COVID vaccine that has been carried out by various pharmaceutical firms to save human society from this pandemic.
What makes CTMS a staunch system?
Clinical Trial Management Software (CTMS) helps to organize clinical trials from all regards including collecting patient data, scheduling appointments, data reporting, analysis of records, and data management.
An efficient CTMS that usually includes most or all of these features:
- Account Management
- Investigator Database Management/portal
- Study Management
- Budget Management
- Site Management
- Subject Enrollment
- Subject Visits
- Monitoring Reports
- Project Management
- Protocol Events
- Adverse Events
- CRF/eCRF
- eTMF
- Drug and device Accountability
- Real-time Analytics
- eCOA/ePRO
In addition to that, a sound CTMS can leverage the features of third-party EDC systems like IBM Merge, Medidata RAVE, ClinCapture, OpenClinica, RedCap, etc which makes it a flexible and powerful tool in the market.
New technologies will always drive the businesses to make the most out of it. With CTMS specific to CTMS, it is essential nowadays to have the system compatible with the EDC to bring the study-specific details to the clinical trial systems that can help to reduce the time and cost and also accelerate the processes to bring the therapies to the market faster.
CTMS & IBM Merge
IBM Clinical Development, a cloud-based data management platform that has Electronic Data Capture (EDC) in place. The platform offers solutions that cover the entire clinical study – from startup to submission. It helps teams to gather, analyze, and manage data with deep analytics irrespective of the size and complexity.
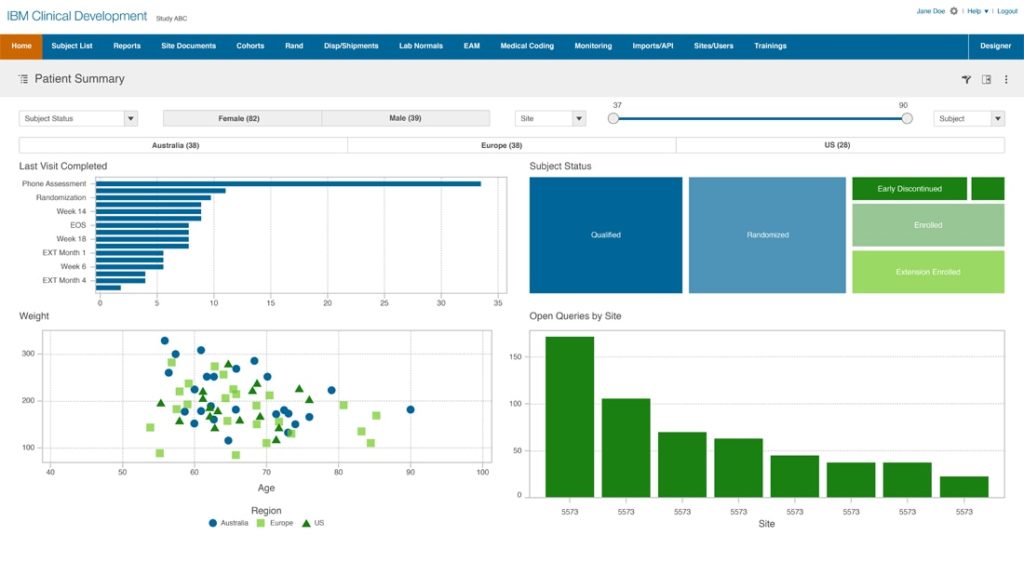
The integration of a CTMS with the IBM Clinical Development can bring in the data in a hassle-free way and map the data across various entities to remodel it and visualize it in a coherent view.
Some of the aspects of the IBM Clinical Development include patient engagement, medical coding, data integration, reporting, and analytics.
Business Use Cases:
Cloudbyz CTMS is used to manage efficient and seamless clinical operations and EDC systems are used for accurate clinical data capture.
-
- Subjects
- As subjects are screened and enrolled, subject data is brought over from EDC to CTMS to track enrollment metrics including screened, screen-failed, and successfully enrolled, to make sure subject enrollment is on track to meet the planned target and milestones.
- Subjects data is also used in CTMS to track enrollment rate, screen failure rate, and generating payments for each subject enrollment and screen failure.
- Subject data in CTMS is also used during monitoring activities to track subjects monitored as part of the trip reports which will be filed in eTMF.
- Subjects
- Subject Visits and Procedure
-
-
- Upon successful enrollment, subject visit and procedure data are brought over to CTMS for generating payments associated with each visit and procedure.
- Visits and Procedure data are also used in CTMS as part of monitoring and trip reports.
-
- eCRF
-
-
- eCRF data is brought over to CTMS from EDC to generate payments associated with each eCRF completion.
- eCRF data is also used for source data verification (SDV) during monitoring visits and generation of trip reports.
-
- Adverse Events
-
-
- Bringing Adverse Events into CTMS from EDC helps the clinical operations team to keep a close tab on tracking and response planning. Via automated workflow in CTMS, the clinical operations team will be able to deploy rapid response plans and report Adverse Events to sponsors and regulatory agencies promptly and file all Adverse Events in eTMF with a click of a button.
- Having Adverse events in CTMS helps in risk management as well.
-
- Protocol Deviations
-
- Having visibility to protocol deviations in CTMS helps the clinical operations team to quickly deploy response planning including corrective action and preventive (CAPA) by leveraging automated workflow and task assignments. Track and report as part of monitoring reports and notify sponsors and regulatory agencies.
-
Integrated CTMS and EDC help in enabling end-to-end process automation and real-time visibility and insight help Sponsors, CRO, and Sites to collaborate systematically and constructively across clinical research processes and helps improve quality, increase efficiency and plummeted cost and time throughout the clinical research.
How can the two systems interlace?
Cloudbyz CTMS can make a connection with the IBM Merge through the following steps:
- Callout to get the access token.
- Check the callout response.
- Callout to get the list of subjects.
- Map the subjects in the Salesforce system.
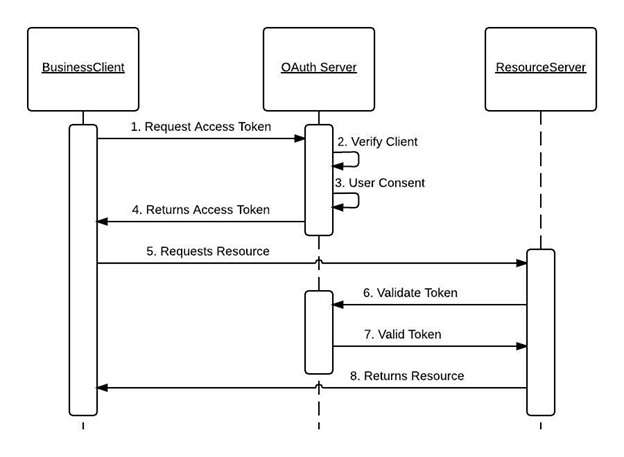
With the help of OAuth 2.0, the clinical trial system can authenticate with the IBM Clinical development and grant access to register it as a trusted third-party system. If authorization is granted, we can use callouts to fetch the response from the IBM Merge which makes it easy for the gathering of study details.
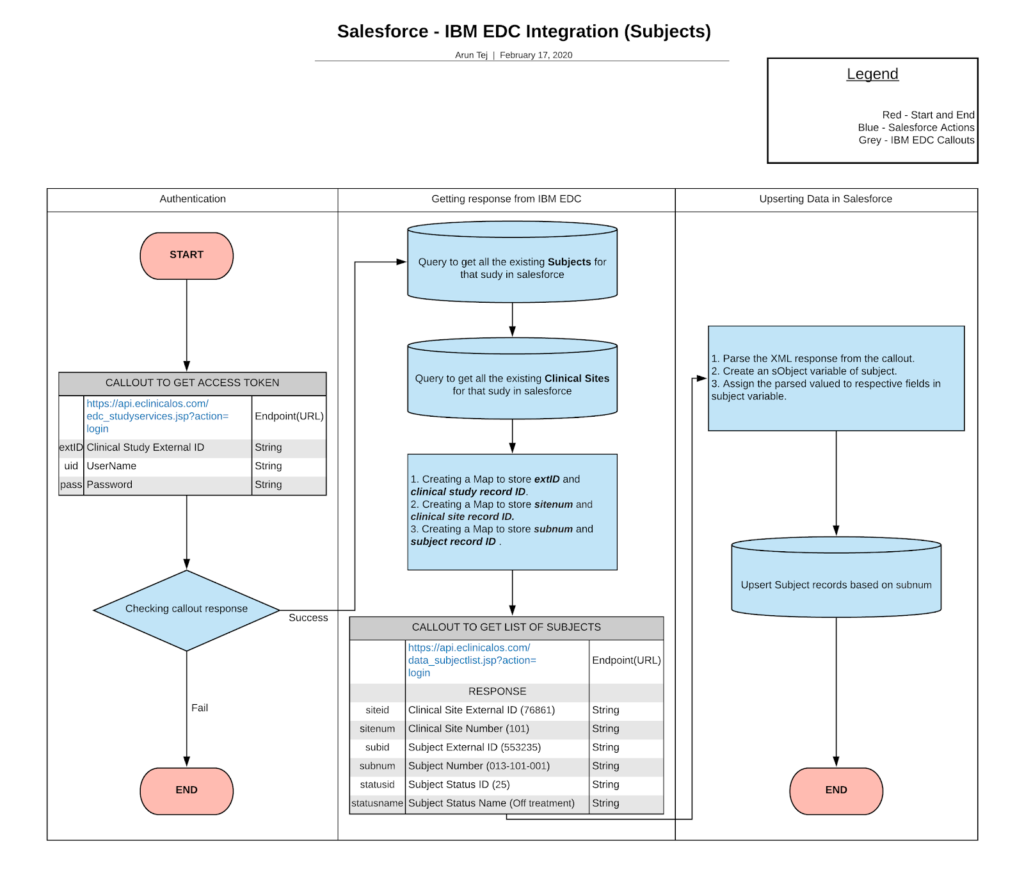
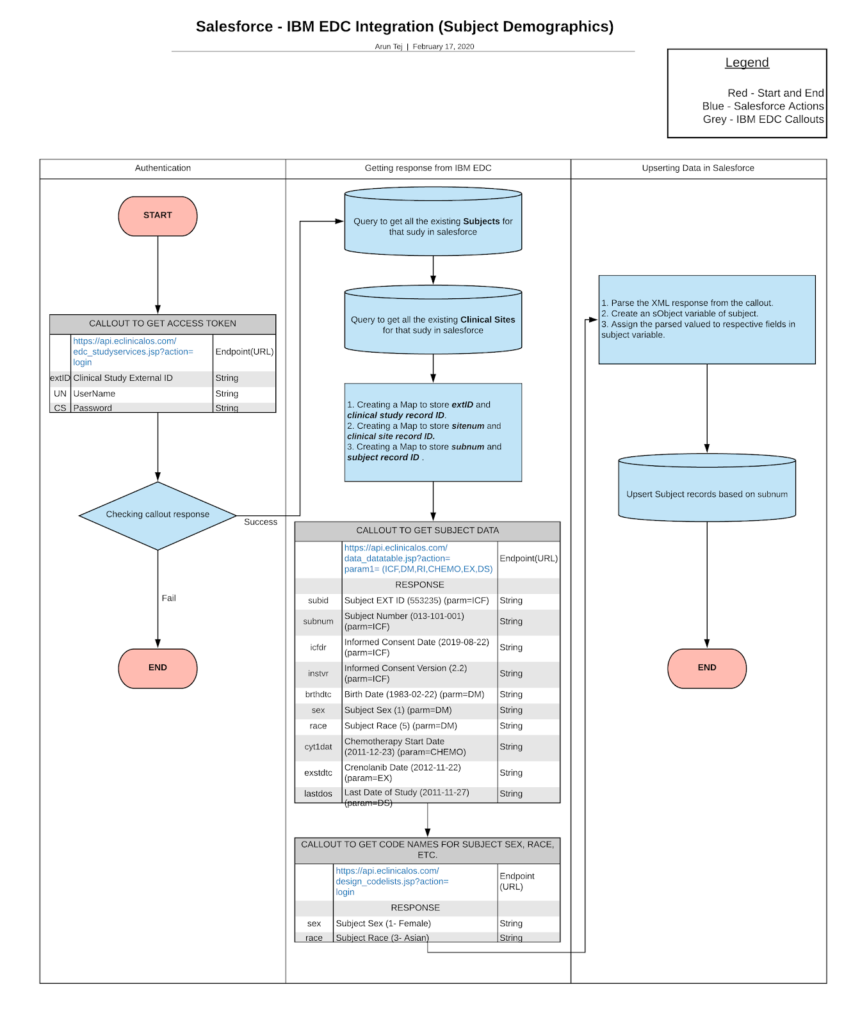
Fetching Subject data from IBM Merge:
IBM Merge can help companies for early-phase trials i.e, study resources, case report forms. Also it provides the ability for the systems to capture data accurately and present it to users quickly.
For fetching the subject data from EDC to the clinical trials, the Salesforce system (CTMS) will have to request the required parameters as follows.
-
- Request URL
- Request Type
- Request Header
- Auth Code that is obtained from the OAuth 2.0 Authorization.
The final word is,
Adding new technologies to the system should accelerate the efficiency and lighten the time complexity and cost factor. But most of the time, it is happening the other way around. Advanced technologies, when added to the existing systems, result in many complications. So, after deep analysis and assessments, a sound CTMS product can leverage the technical services provided by the IBM Merge to make the CTMS more flexible and compatible with no dawdles.