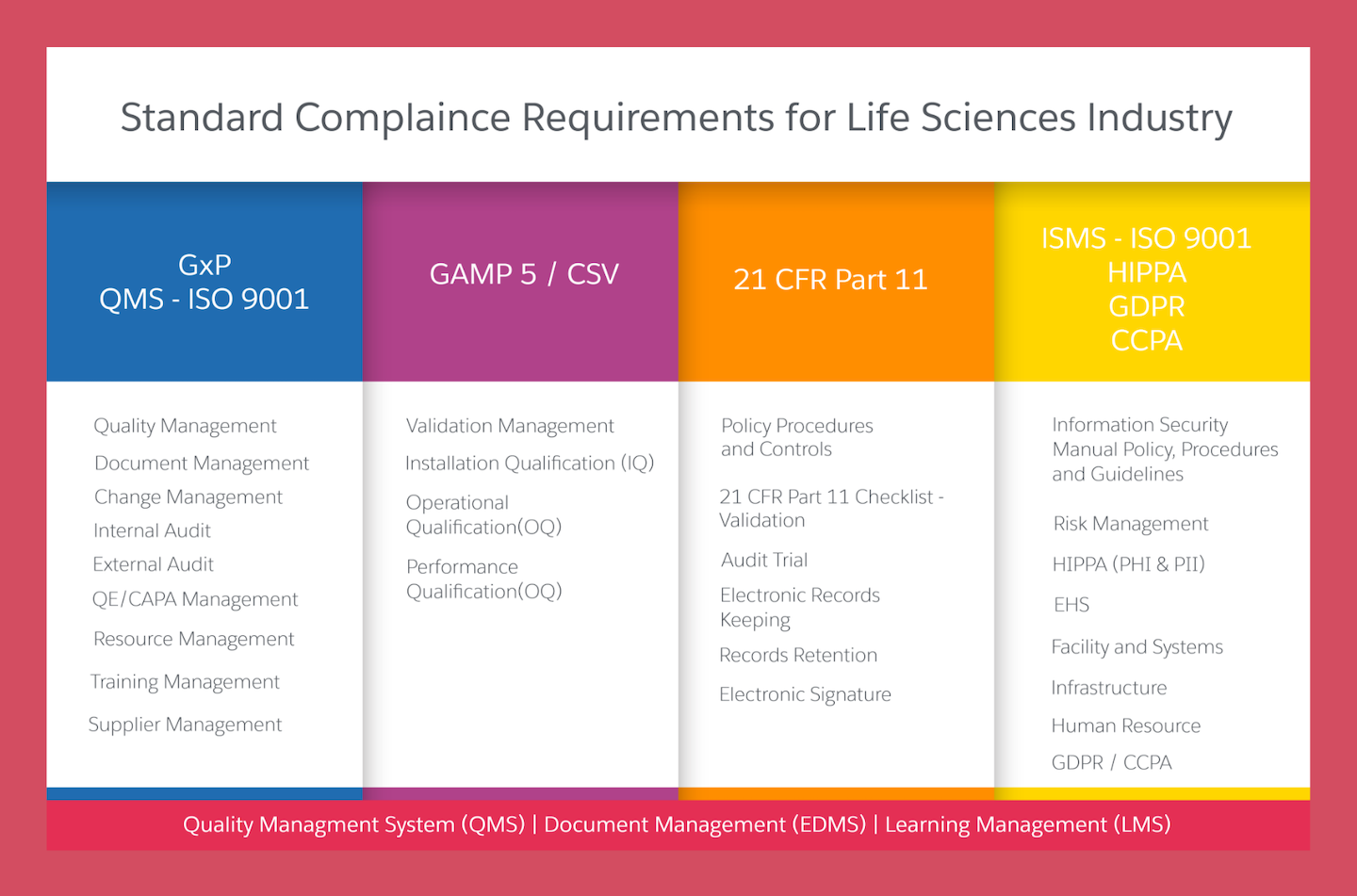
Our Implementation
Life Sciences Organizations engaged in drug development face an increasingly challenging environment: an expanding global footprint for clinical trials, evolving regulatory requirements, dynamic partnership models, and rapidly evolving technology. At the same time, many organizations are also innovating how trials are designed and conducted, adding to the complexity. Managing this complexity could be greatly enhanced by an effective and efficient Quality Management System (QMS). However, what constitutes an effective and efficient QMS is not well defined for clinical development. Cereblis QMS Consulting Services helps to develop a flexible, holistic, clinical QMS framework to address this gap and helps Quality Management System Design, Gap Analysis, Implementation, and Improvement.
Cereblis Consulting Services for the design, development, and implementation of a Quality Management System (QMS) aims to help sponsors and CROs develop a culture of risk prevention rather than one of managing issues.
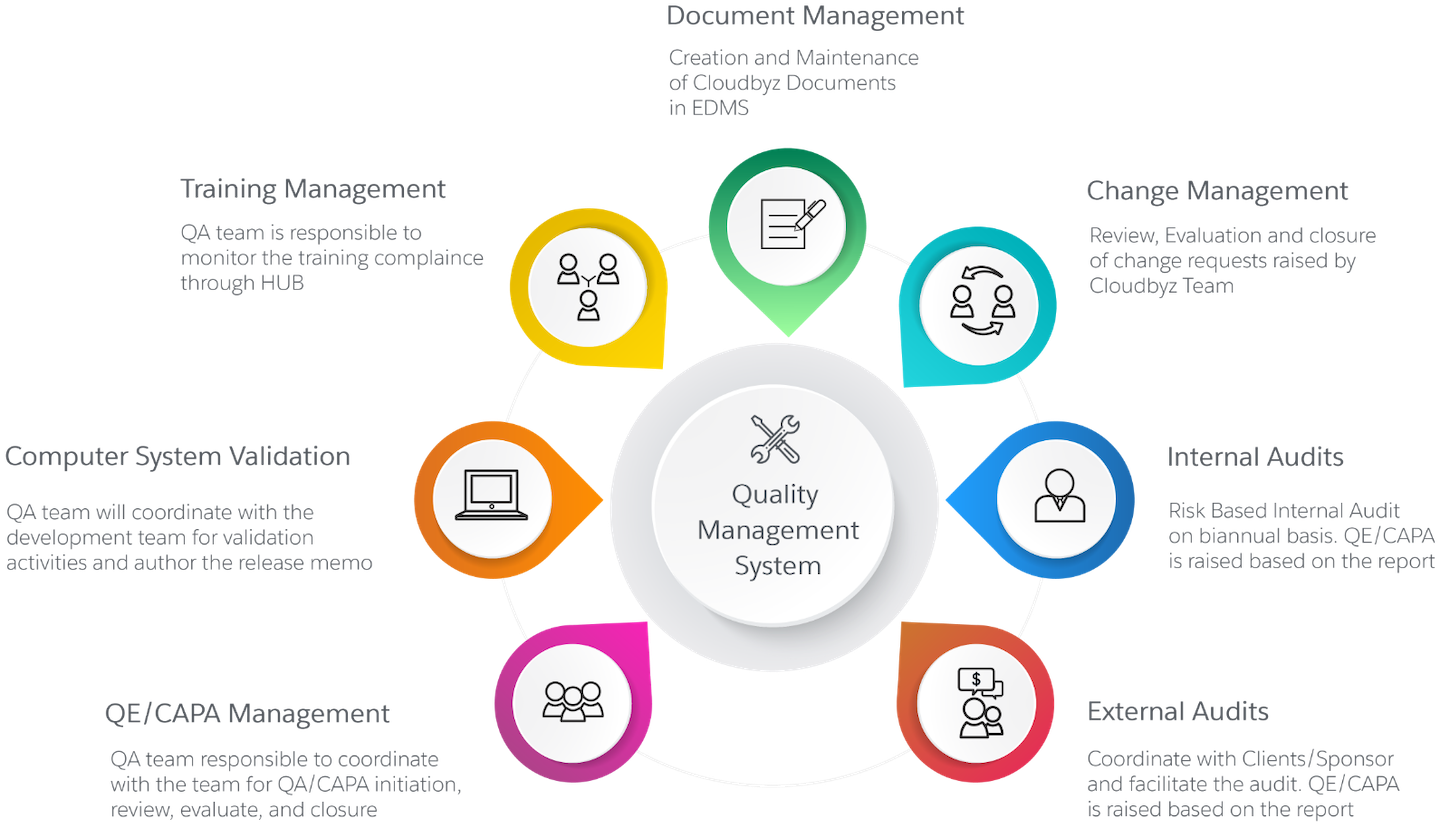
Quality Management Process
- Document Management
- Change Management
- Internal Audits
- External Audits
- QE/CAPA Management
- ComputerSystem Validation
- Training Management
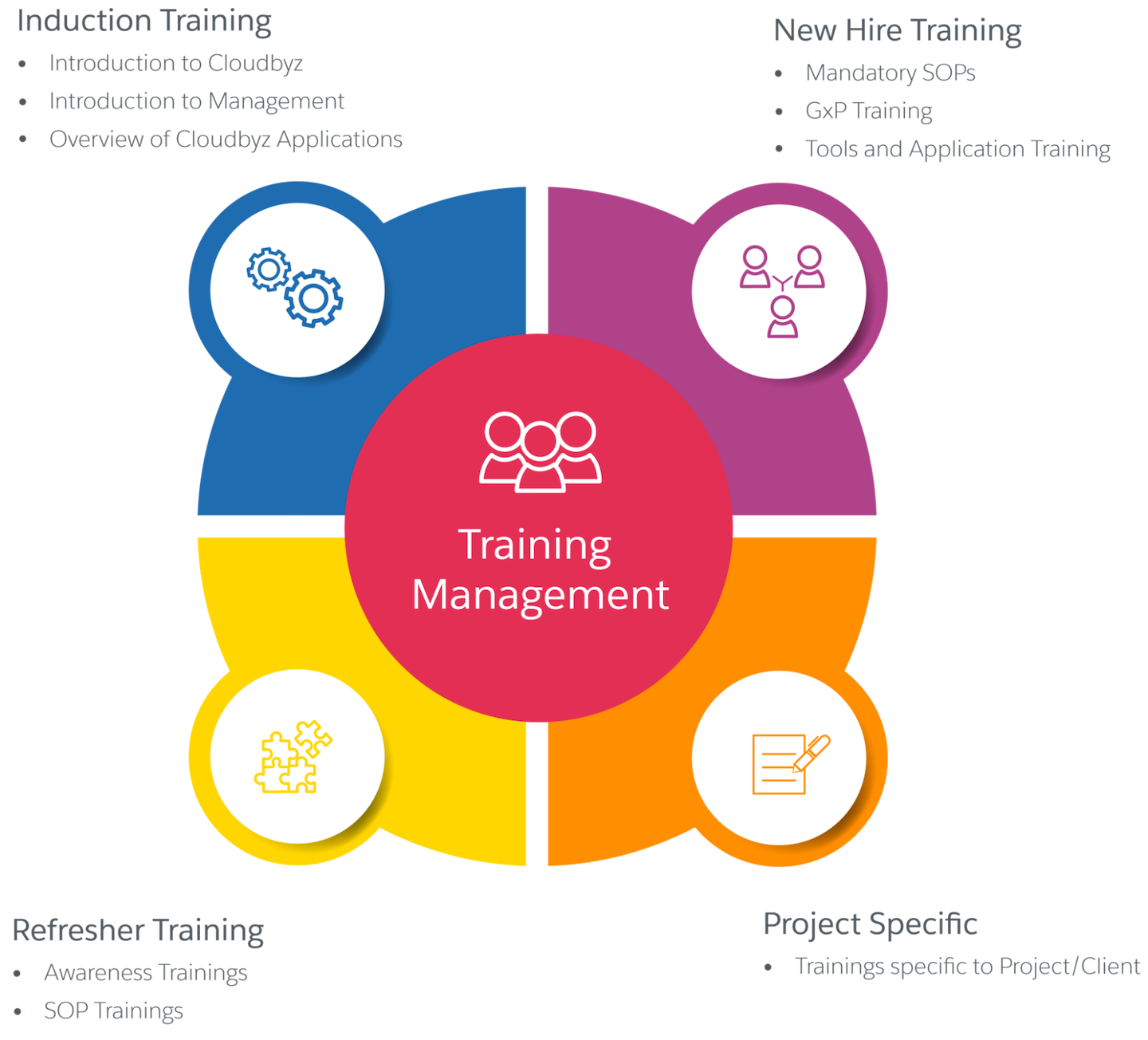
Training Management
A proactive, risk-based approach ensures patient safety, improves data quality, provides data integrity, and increases inspection preparedness.

Why Quality Management System is lmportant?
To be compliant with ICH E6 (R2), risk-based quality management for clinical trials is required. A QMS is a framework for all activities, including quality control, quality assurance, quality improvement, and the reporting of these activities within an organization.
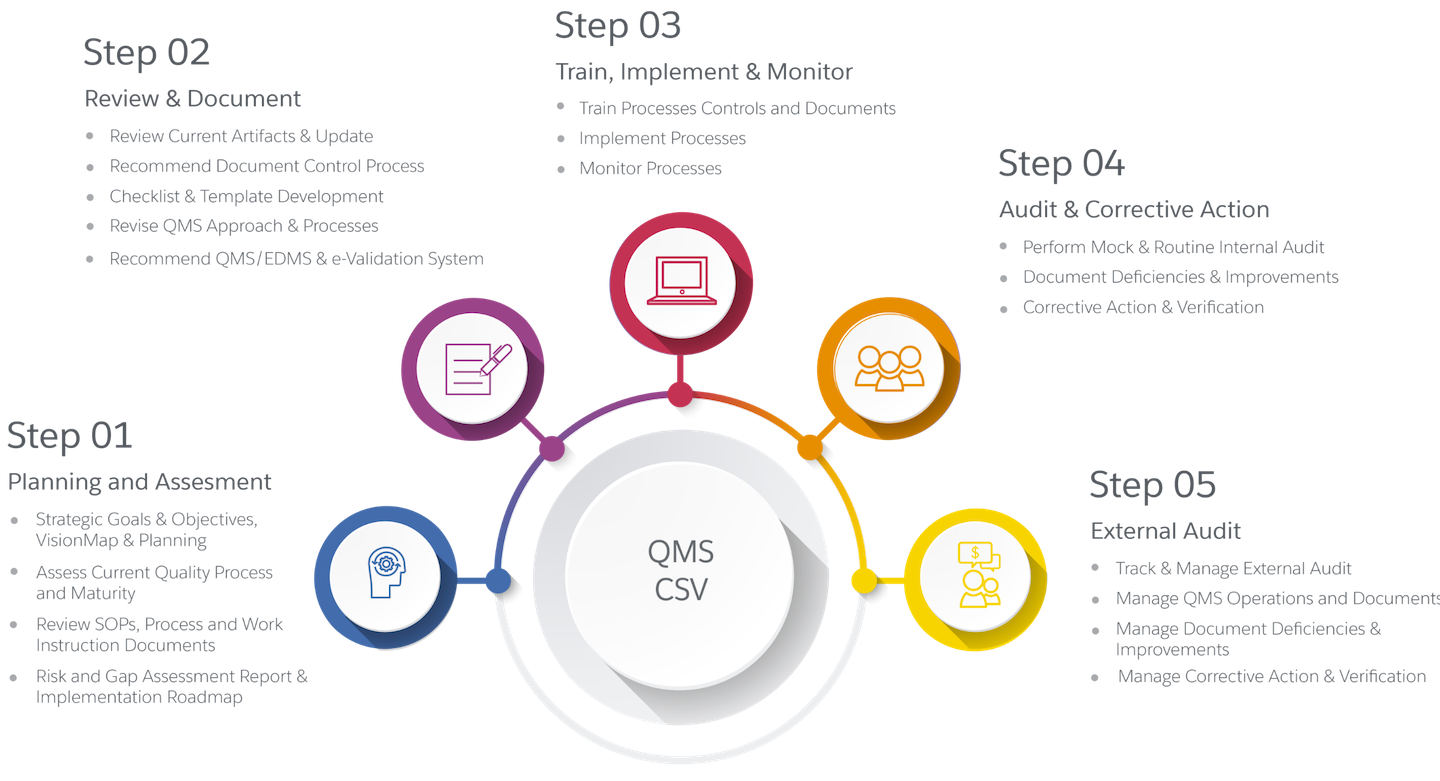
QMS lmplementation Approach
We alleviate time, staff, and other resource constraints that prevent compliance and preparedness assurance — allowing teams to focus on their core responsibilities. To simplify and streamline the successful implementation of company’s QMS, we leverage the quality management and inspection readiness leading practices, tools and standards, developed and refined by Cereblis QMS Practice.
